- HOME
- Technical Information
- Commissioned Analysis and Research
- X-Ray Fluorescence Spectrometry (XRF)
X-Ray Fluorescence Spectrometry (XRF)
Principle
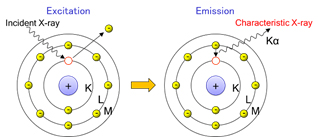
X-ray fluorescence spectrometry is a method that determine elements both qualitatively and quantitatively by measuring characteristic X-rays (fluorescent X-rays) generated when X-rays (primary X-rays) irradiate a sample.
Electrons in the K-shell is ejected from the atom by an external primary excitation X-ray, and vacancies are created. When electrons from the outer shell transition to fill these vacancies, characteristic X-rays corresponding to the energy difference are generated.
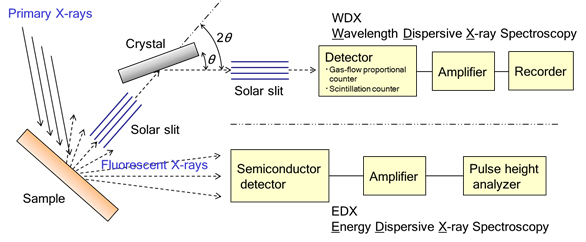
● Wavelength-dispersive X-ray fluorescence (WDX)
Characteristic X-rays are detected after dispersing by a analyzing crystal.
nλ = 2d sinθ : Bragg condition
The elements from Boron (B) to Uranium (U) are measurable.
WDX provide better resolution than EDX.
● Energy-dispersive X-ray fluorescence (EDX)
Characteristic X-rays are detected with a semiconductor detector, and elements from Sodium (Na) to Uranium (U) are measurable.