- HOME
- Technical Information
- Commissioned Analysis and Research
- Vapor Pressure
Vapor Pressure
Principle
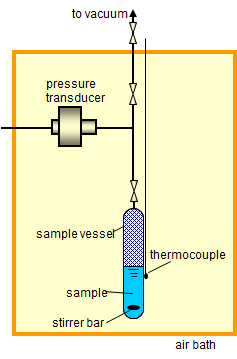
We have various vapor pressure measurement methods. Figure 1 and table 1 show the characteristics of the vapor pressure measurement method. Appropriate method of the vapor pressure measurement is determined by considering the properties of the sample, the amount of the sample, and the magnitude of the vapor pressure. Figure 2 shows a schematic diagram of static method. This method measures the equilibrium vapor pressure of a sample directly with a pressure gauge.
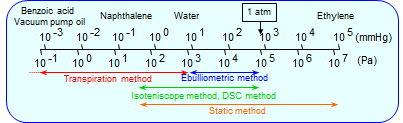
Table 1 Features of vapor pressure measurement method
| Material | Sample amount | Accuracy | Features | |
|---|---|---|---|---|
| Static method | Liquid | ca. 30 mL | ±5 % | ・High pressure measurement ・Long - term measurement ・Mixtures can also be measured |
| Ebulliometricmethod | Liquid | ca. 150 mL | ±5 % | ・Short - term measurement ・Mixtures can also be measured |
| Isoteniscope method | Liquid | ca. 10 mL | ±2~3 % | ・Small amount of sample ・Susceptible to errors of impurities and decomposition products ・Mixtures can’t also be measured |
| Transpiration method | Liquid Solid | ca. 1 g | ±10~20% | ・Solid sample can be measured ・Low vapor pressure (ca. 0.1 Pa) can be measured ・Low accuracy ・Need molecular weight |
| DSC method | Liquid Solid | ca. 1 g | ±10~20% | ・Rapid measurement ・Low accuracy ・Mixtures can’t also be measured |