- HOME
- Technical Information
- Commissioned Analysis and Research
- UV-Vis (Ultraviolet-Visible) Spectroscopy
UV-Vis (Ultraviolet-Visible) Spectroscopy
Principle
The electronic transition energy between molecular orbitals (HOMO-LUMO) and band gap energy in semiconductors often lie in the UV-visible region. Therefore, information on the electronic state of a sample can be obtained from the UV-visible absorption spectrum. Especially, the absorption spectrum in the visible region corresponds to the color of the sample. Since the absorption intensity is proportional to the sample concentration, the quantitative analysis can be possible.
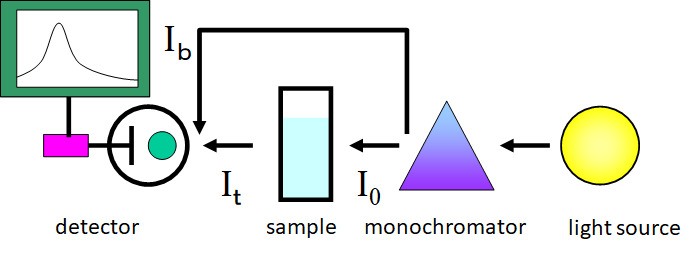
An optical schematic of a double-beam spectrometer is shown below. After the monochromator, the light is divided into the light (Ib), which is used to monitor the fluctuation of the light intensity, and the light (I0), which is used to measure the absorption of the sample. The light passing through the sample (It) and Ib are detected alternately. In this double-beam method, the fluctuation of the light source is corrected sequentially, so that the absorption intensity can be measured stably and accurately.
| Measurement method | Example |
| Transmittance Specular reflectance Diffuse reflectance |
Color and degradation analysis Band gap analysis |