- HOME
- Technical Information
- Commissioned Analysis and Research
- CHN elemental analysis
CHN elemental analysis
Principle
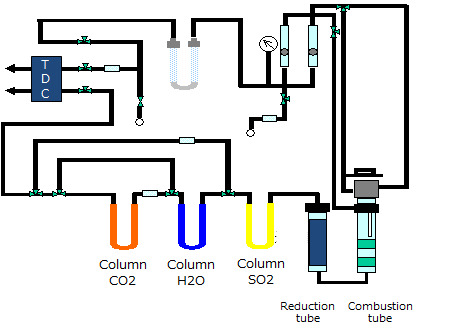
A sample in a tin boat or tin capsule is weighed accurately and burned in a combustion tube with oxygen. The produced gas mixture (CO2, H2O, NOx, etc.) is introduced into the reduction tube, where nitrogen oxide is converted to N2 gas. Then the N2 gas is detected by TCD, on the other hand, H2O and CO2 are trapped on column. After N2 detection, the column (CO2) is heated and evolved CO2 gas is detected by the same TCD. Next the column (H2O) is heated and evolved H2O gas is detected by TCD. The amount of each element is determined from the peak area obtained by the calibration curve prepared from the standard sample.